Details of the Drug
General Information of Drug (ID: DMZLGQV)
| Drug Name |
AZD2624
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Pavinetant; AZD-2624; MLE4901; 941690-55-7; UNII-3U471ZVC5K; 3U471ZVC5K; AZ124752520; pavinetantum; Pavinetant [USAN]; SCHEMBL3587478; GTPL5775; CHEMBL3545233; CHEBI:140478; QYTBBBAHNIWFOD-NRFANRHFSA-N; BDBM50180193; AKOS032946112; DB11692; CS-7979; 4-Quinolinecarboxamide, 3-((methylsulfonyl)amino)-2-phenyl-N-((1S)-1-phenylpropyl)-; HY-14432; KB-74807; 3-methanesulfonamido-2-phenyl-N-[(1S)-1-phenylpropyl]quinoline-4-carboxamide; 3-[(methanesulfonyl)amino]-2-phenyl-N-[(1S)-1-phenylpropyl]quinoline-4-carboxamide
|
||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||
| Structure |
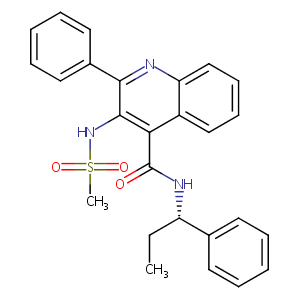 |
||||||||||||||||||||||||||
| 3D MOL is unavailable | 2D MOL | ||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 1 | Molecular Weight (mw) | 459.6 | |||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 5.1 | ||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 7 | ||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 5 | ||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| ICD Disease Classification | 06 Mental, behavioural or neurodevelopmental disorder | |||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Disease Class | ICD-11: 6A20 Schizophrenia | |||||||||||||||||||||||||||||
| The Studied Tissue | Pre-frontal cortex | |||||||||||||||||||||||||||||
| The Studied Disease | Schizophrenia [ICD-11:6A20] | |||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||||||||
References
